2021年05月22日
总结篇 | Biofuture2021

为期两天的Biofuture 2021 下一代抗体药技术创新大会&细胞与基因治疗创新大会圆满落下了帷幕。此次展会以“生物药未来技术创新”为主题,邀请了生物制药领域学术界和产业界的专家、学者、企业家、创新者和创业者齐聚一堂。大会涵盖了抗体药物机会与挑战、抗体药物创新开发及生产工艺优化、细胞基因治疗新突破、细胞与基因治疗产业创新与商业化等专题内容,来访者一同互动学习交流发表见解。
精彩回顾 | 01

康晟生物位于【#B21】展台摆展,本次除了向大众展示康晟生物主打产品、技术服务、技术咨询等内容外,还与各界同行围绕着抗体药物领域、基因治疗领域产业创新、商业化等进行了密切的交流。不仅了解到了行业内外最新的动态,还了解到了客户的有关需求。通过广泛的沟通及探讨让各位同行对康晟产品、授权、服务理念等内容有了更直观的了解。截止至20日下午16时,接待咨询人数总计百余位、调查问卷累计回收100+、累计发放各产品手册200+。
QuaCell明星产品QuaMono™ Plus 单克隆培养基受到热捧,二十个限量体验名额被迅速抢空;解决细胞与基因治疗客户痛点的HEK293宿主细胞株平台及商业化授权同样备受关注,现场接受多家企业咨询,达成授权意向2个;Wayne293瞬转培养基及其他培养基也派发数十份试用。
精彩回顾 | 02
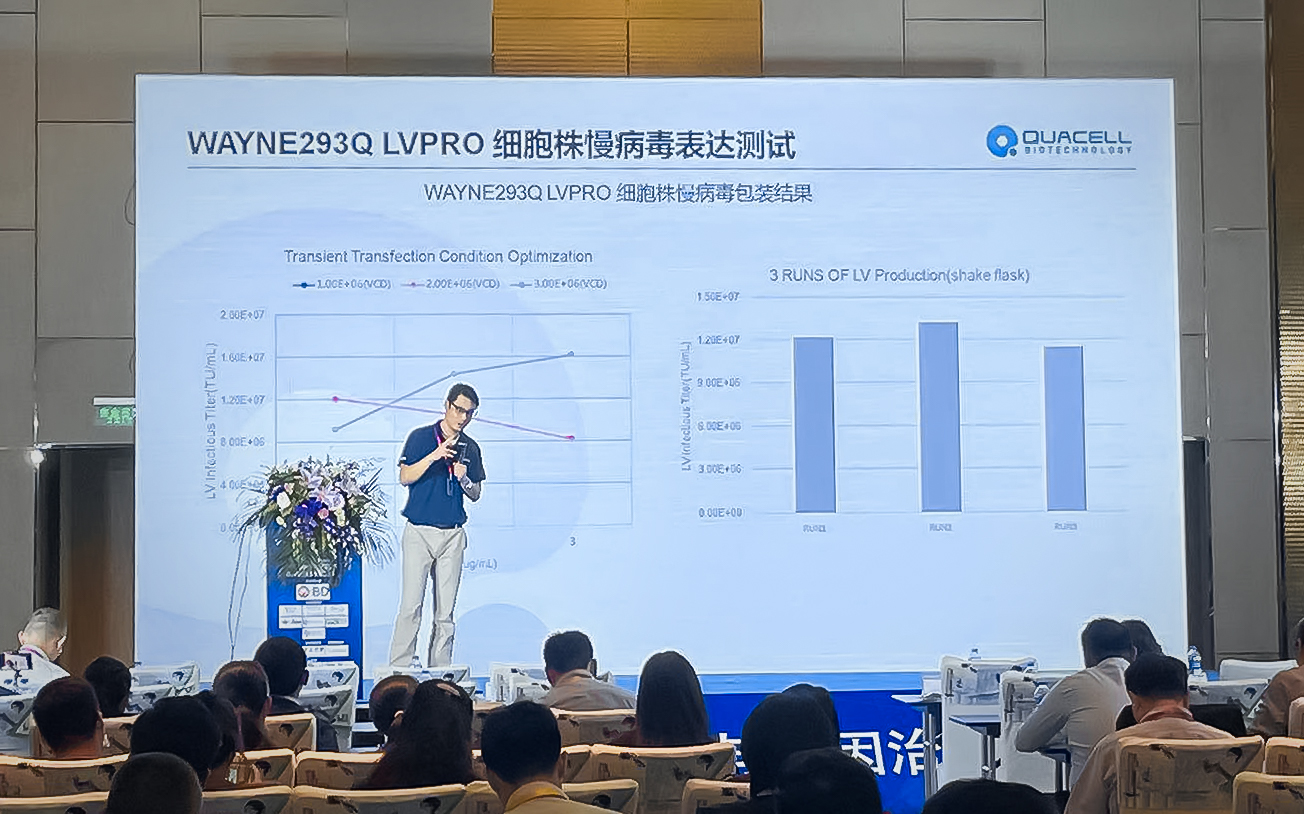
随着全球病毒载体疫苗和基因/细胞治疗研究的逐步深入,HEK293及其衍生细胞系现已广泛用于学术研究以及全球制药和生物技术行业。康晟生物作为HEK293细胞系中国区域独家的授权商,致力于为细胞与基因治疗合作伙伴,一站式地解决293细胞进口、溯源、授权及病毒包装产业化问题,并开发出更多的基因治疗与细胞治疗开发工具,助力生物制药产业化进程。望眼生物药技术发展态势及创新药物的不断涌现,“生物药未来技术创新”充满着机遇与挑战,康晟生物也将秉承着更专业的研究、更高效的服务、更优质的产品,立足既有优势,谋划发展,共同迈向生物医药的未来。